As You Move Across the Periodic Table
The removal of the electron requires 13120 kJmol of energy. Due to this the atomic radius decreases.

Trends In Periodic Table Chemistry Education Periodic Table Chemistry
This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell.

. The size of an elements ionic radius follows a predictable trend on the periodic table. In atoms the number of protons equals to the number of electrons too if the. The atoms have less electrons.
Fluorine can form ionic bonds with. Also to know is why does ionization energy increase across. The answer should be 2 electrons.
The atoms have less mass. Which statement correctly and completely identifies a trend. A Atomic radii increase.
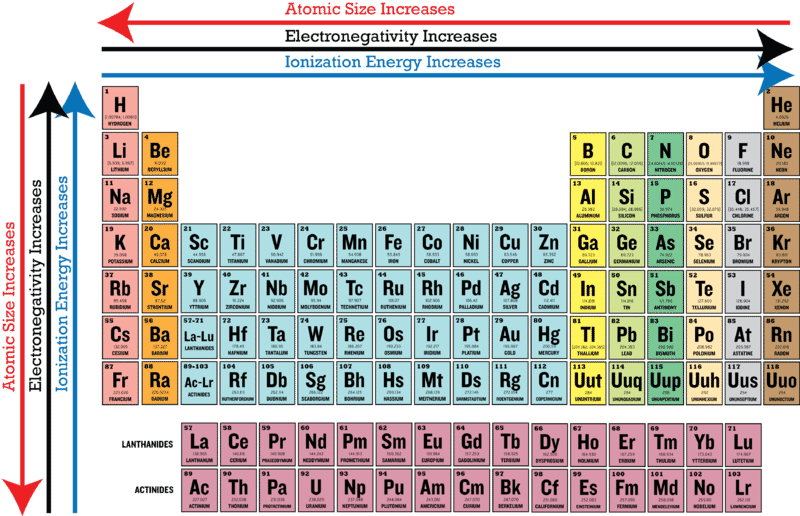
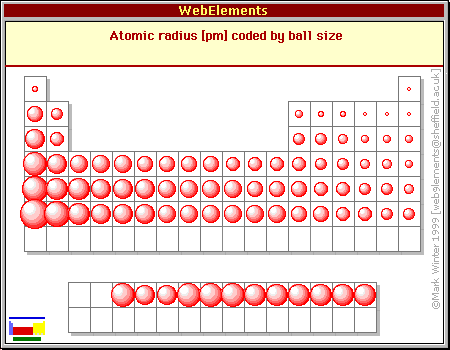
More electrons in an orbital lowers energy. As you move from left to right across the periodic table elements. Atomic radius increases moving left to right across a period and decreases from top to bottom.
The atoms have less mass. The atoms have less electrons. What is the location of elements in the periodic table related to.
Why radius of Na is smaller than na. There are trends in metallic character as you move across and down the periodic table. Metallic character decreases as you move across a period in the periodic table from left to right.
As you move across a period in the periodic table the types of commonly encountered bonding interactions change. Because as you move to the right of the periodic table each element gets a proton added to its nuclei an what this doesnot its only use obviouslyis it increases the attraction between the nuclei and its electrons which shrinks the atom and due to there being greater attraction and a smaller radii this increases the attraction between the nucleus of an atom and the valence. As you move across the periodic table atoms tend to get smaller because.
The ionization energy increases because as you move across the periodic table the atomic radius decreases. Metallic character increases as you move. A higher atomic number.
How was Mendeleevs periodic table arranged. As you move across the periodic table atoms tend to get smaller because ______________. The ionization energy of the elements will _____ as you move from left to right across a period on the periodic table.
As you move across the periodic table atoms tend to get smaller because. By increasing atomic mass. 1-As we move from left to right across the periodic table what is the general trend.
As you move across the periodic table atoms tend to get smaller because _____. Fluorine is the most electronegative element. As you move from left to right across the periodic table atoms have a greater nuclear charge and a smaller covalent radius.
The atoms have more mass. Electronegativity increases as you move across the periodic table from left to right. While this is the basic definition of the electronegativity trend to truly understand it it would be helpful to put it in perspective and look at some specific examples of the trend.
Check out a sample QA here. Because the pharmaceutical industry features a high rate of. Want to see the full answer.
This occurs due to a greater charge on the nucleus causing the electron bonding pairs to be very attracted to atoms placed further right on the periodic table. Why is iodine larger than bromine. A corollary to this is that because there are more electrons per atom as the atomic number increases the atomic radius.
This allows the nucleus to attract the bonding electrons more strongly. This trend is seen as you move across the periodic table from left to right. Increase This is because the electrons are all the same distance from the nucleus in the same period but the elements have ______ protons as you move from left to right across the periodic table.
Ionic radius decreases moving from left to right across a row or period. Atomic radius decreases moving from left to right across a period and increases from top to bottom. Smaller nuclear charge lowers energy.
- Atomic radius decreases across a period and increases down a group. The electronegativity increases while it decreases as you move down a group of elements. The elements in the periodic table are arranged by increasing atomic number which is also the number of protons in an element.
As you move from up to down in a column of the periodic table elements have. As you move down a column or group the ionic radius increases. Is a successful drug manufacturer.
Next Fang and Bone Corp. As you move across the periodic table atoms tend to get smaller because ______________. - the atoms have more protons.
Why does electronegativity increase going from left to right across the second row of the periodic table until just before you reach neon. The valence electrons are more strongly attracted by the nucleus. For example Carbon has 6 protons it is the sixth element on the table.
C Nuclear shielding increases. As you move across the periodic table from left to right A do the atoms get smaller or larger. Atomic radius increases diagonally across the periodic table.
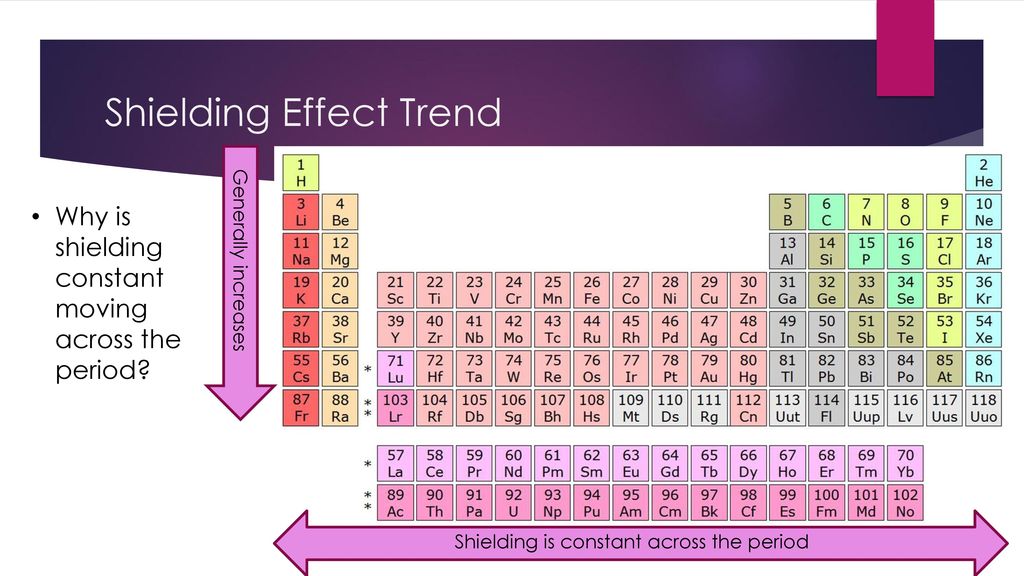
While Nitrogen which has 7 protons is after Carbon. C are the metals becoming more or less reactive. This increases the effective nuclear charge due to poor shielding.
- iodine has more energy levels and greater shielding effect than bromine How many valence. D Metallic character decreases. Since the atomic radius decreases the outermost electrons valence electrons are held more tightly to the increasing number of protons in the nucleus.
The atomic radius increases as you move down a column because for every new row of the table a new electron shell is added to the atom. For example at the beginning of Period 2 elements such as lithium and beryllium form only ionic bonds in general. B are the ionization energies increasing or decreasing.
The atoms have more protons. This is because each row adds a new electron shell. When you move from left to right across a period or row of the periodic table atomic radius decreases as more and more electrons are added to same energy level.
The atoms have more protons and the electrons are on the same energy level. Moving across the period elements such as boron carbon nitrogen and oxygen tend to form covalent bonds. The atoms have more mass.
The atomic radius for atoms of an element tends to go up as you move down a group of elements in the table.

Color And Learn About The Periodic Table Layers Of Learning Physical Science Middle School Teaching Science Physical Science

Understanding The Periodic Table Through The Lens Of The Volatile Group I Metals

Various Trends In The Periodic Table With Examples Periodic Table Trending Infographic

Chart Summarizes The Major Trends In The Properties For Elements In The Periodic Table Trends Electr Chemistry Lessons Chemistry Classroom Teaching Chemistry
Periodic Trends In Electronegativity Ck 12 Foundation
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

Mrs Sharon King Integrated Science I Teaching Chemistry Chemistry Classroom Chemistry Education

Periodic Table Trends Atomic Size Melting Boiling Point Trend

Image Result For Periodic Table Of Business Process Management Business Process Management Software Development Knowledge Management System

Periodicity Trends In The Periodic Table Chemistry Classroom Teaching Chemistry Chemistry Lessons

Periodic Game Of The Elements Games Board Games Strategy Board Games

Engaging Students In Learning About Force And Newton S Laws Of Motion Physical Science Experiments Nonfiction Articles Newtons Laws

Free Printable Periodic Table Of The Elements 11 Page Set Of Worksheets Science Printables Teaching Chemistry Middle School Science

Chemistry Periodic Table Trends Guided Inquiry Lesson Chemistry Periodic Table Chemistry Lesson

Polarity Is Caused By The Electronegativity Of Atoms Electronegativity Is A Measure Of An Atoms Ability To Attract Molecular Shapes Covalent Bonding Molecular

Periodic A Game Of The Elements Games Strategy Board Games Science Games
How Does Atomic Radius Change From Left To Right Across A Period In The Periodic Table Socratic

Elements Wlonk Com Periodic Table Of The Elements Chemistry Classroom Teaching Chemistry
Comments
Post a Comment